
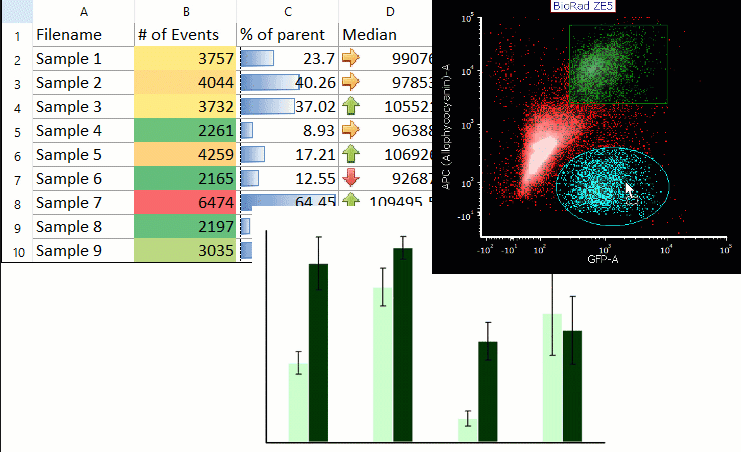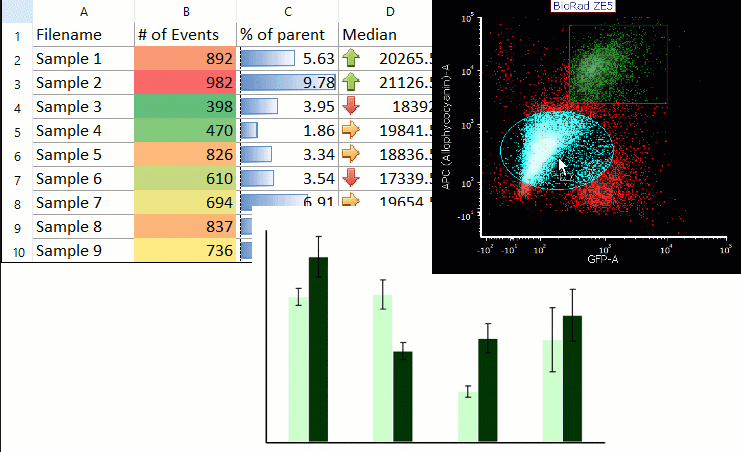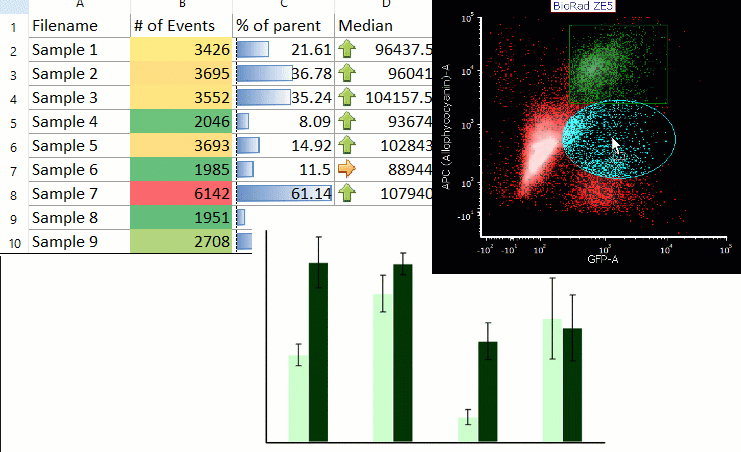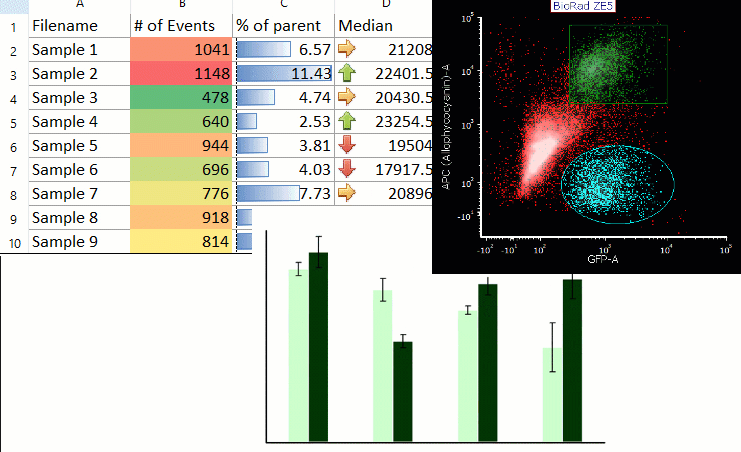
Acoustophoresis allowed to effectively separate PDX cells from spiked PBPC products. Acoustic cell separation did not affect neuroblastoma cell growth. PDX cells and PBPCs showed distinct size distributions, which is an important parameter for efficient acoustic separation. Cells were separated by ultrasound in an acoustophoresis microchip and analyzed for recovery, purity and function using flow cytometry, quantitative real-time PCR and cell culture.
Neuroblastoma patient-derived xenograft (PDX) cells were spiked into PBPC apheresis samples as a clinically relevant model system. Motivated by the potential therapeutic benefit of tumor cell removal as well as the high prognostic and diagnostic value of isolated circulating tumor cells from stem cell grafts, we established a label-free acoustophoresis-based microfluidic technology for neuroblastoma enrichment and removal from peripheral blood progenitor cell (PBPC) products. Graft-contaminating tumor cells correlate with inferior outcome in high-risk neuroblastoma patients undergoing hematopoietic stem cell transplantation and can contribute to relapse.


 0 kommentar(er)
0 kommentar(er)
